 SEEd - Chemistry
SEEd - Chemistry
 Printable Version (pdf)
Printable Version (pdf)
 Course Introduction
Course Introduction
Utah Science with Engineering Education Standards
Utah’s Science and Engineering Education (SEEd) standards were written by Utah educators and scientists, using a wide array of resources and expertise. A great deal is known about good science instruction. The writing team used sources including A Framework for K–12 Science Education1, the Next Generation Science Standards2, and related works to craft research-based standards for Utah. These standards were written with students in mind, including developmentally appropriate progressions that foster learning that is simultaneously age-appropriate and enduring. The aim was to address what an educated citizenry should know and understand to embrace the value of scientific thinking and make informed decisions. The SEEd standards are founded on what science is, how science is learned, and the multiple dimensions of scientific work.
Principles of Scientific Literacy
Science is a way of knowing, a process for understanding the natural world. Engineering applies the fields of science, technology, and mathematics to produce solutions to real-world problems. The process of developing scientific knowledge includes ongoing questioning, testing, and refinement of ideas when supported by empirical evidence. Since progress in modern society is tied so closely to this way of knowing, scientific literacy is essential for a society to be engaged in political and economic choices on personal, local, regional, and global scales. As such, the Utah SEEd standards are based on the following essential elements of scientific literacy.
Science is valuable, relevant, and applicable.
Science produces knowledge that is inherently important to our society and culture. Science and engineering support innovation and enhance the lives of individuals and society. Science is supported from and benefited by an equitable and democratic culture. Science is for all people, at all levels of education, and from all backgrounds.
Science is a shared way of knowing and doing.
Science learning experiences should celebrate curiosity, wonder, skepticism, precision, and accuracy. Scientific habits of mind include questioning, communicating, reasoning, analyzing, collaborating, and thinking critically. These values are shared within and across scientific disciplines, and should be embraced by students, teachers, and society at large.
Science is principled and enduring.
Scientific knowledge is constructed from empirical evidence; therefore, it is both changeable and durable. Science is based on observations and inferences, an understanding of scientific laws and theories, use of scientific methods, creativity, and collaboration. The Utah SEEd standards are based on current scientific theories, which are powerful and broad explanations of a wide range of phenomena; they are not simply guesses nor are they unchangeable facts. Science is principled in that it is limited to observable evidence. Science is also enduring in that theories are only accepted when they are robustly supported by multiple lines of peer reviewed evidence. The history of science demonstrates how scientific knowledge can change and progress, and it is rooted in the cultures from which it emerged. Scientists, engineers, and society, are responsible for developing scientific understandings with integrity, supporting claims with existing and new evidence, interpreting competing explanations of phenomena, changing models purposefully, and finding applications that are ethical.
Principles of Science Learning
Just as science is an active endeavor, students best learn science by engaging in it. This includes gathering information through observations, reasoning, and communicating with others. It is not enough for students to read about or watch science from a distance; learners must become active participants in forming their ideas and engaging in scientific practice. The Utah SEEd standards are based on several core philosophical and research-based underpinnings of science learning.
Science learning is personal and engaging.
Research in science education supports the assertion that students at all levels learn most when they are able to construct and reflect upon their ideas, both by themselves and in collaboration with others. Learning is not merely an act of retaining information but creating ideas informed by evidence and linked to previous ideas and experiences. Therefore, the most productive learning settings engage students in authentic experiences with natural phenomena or problems to be solved. Learners develop tools for understanding as they look for patterns, develop explanations, and communicate with others. Science education is most effective when learners invests in their own sense-making and their learning context provides an opportunity to engage with real-world problems.
Science learning is multi-purposed.
Science learning serves many purposes. We learn science because it brings us joy and appreciation but also because it solves problems, expands understanding, and informs society. It allows us to make predictions, improve our world, and mitigate challenges. An understanding of science and how it works is necessary in order to participate in a democratic society. So, not only is science a tool to be used by the future engineer or lab scientist but also by every citizen, every artist, and every other human who shares an appreciation for the world in which we live.
All students are capable of science learning.
Science learning is a right of all individuals and must be accessible to all students in equitable ways. Independent of grade level, geography, gender, economic status, cultural background, or any other demographic descriptor, all K–12 students are capable of science learning and science literacy. Science learning is most equitable when students have agency and can engage in practices of science and sense-making for themselves, under the guidance and mentoring of an effective teacher and within an environment that puts student experience at the center of instruction. Moreover, all students are capable learners of science, and all grades and classes should provide authentic, developmentally appropriate science instruction.
Three Dimensions of Science
Science is composed of multiple types of knowledge and tools. These include the processes of doing science, the structures that help us organize and connect our understandings, and the deep explanatory pieces of knowledge that provide predictive power. These facets of science are represented as “three dimensions” of science learning, and together these help us to make sense of all that science does and represents. These include science and engineering practices, crosscutting concepts, and disciplinary core ideas. Taken together, these represent how we use science to make sense of phenomena, and they are most meaningful when learned in concert with one another. These are described in A Framework for K–12 Science Education, referenced above, and briefly described here:
Science and Engineering Practices (SEPs):
Practices refer to the things that scientists and engineers do and how they actively engage in their work. Scientists do much more than make hypotheses and test them with experiments. They engage in wonder, design, modeling, construction, communication, and collaboration. The practices describe the variety of activities that are necessary to do science, and they also imply how scientific thinking is related to thinking in other subjects, including math, writing, and the arts. For a further understanding of science and engineering practices see Chapter 3 in A Framework for K–12 Science Education.
Crosscutting Concepts (CCCs):
Crosscutting concepts are the organizing structures that provide a framework for assembling pieces of scientific knowledge. They reach across disciplines and demonstrate how specific ideas are united into overarching principles. For example, a mechanical engineer might design some process that transfers energy from a fuel source into a moving part, while a biologist might study how predators and prey are interrelated. Both of these would need to model systems of energy to understand how all of the features interact, even though they are studying different subjects. Understanding crosscutting concepts enables us to make connections among different subjects and to utilize science in diverse settings. Additional information on crosscutting concepts can be found in Chapter 4 of A Framework for K-12 Science Education.
Disciplinary Core Ideas (DCIs):
Core ideas within the SEEd Standards include those most fundamental and explanatory pieces of knowledge in a discipline. They are often what we traditionally associate with science knowledge and specific subject areas within science. These core ideas are organized within physical, life, and earth sciences, but within each area further specific organization is appropriate. All these core ideas are described in chapters 5 through 8 in the K–12 Framework text, and these are employed by the Utah SEEd standards to help clarify the focus of each strand in a grade level or content area.
Even though the science content covered by SEPs, CCCs, and DCIs is substantial, the Utah SEEd standards are not meant to address every scientific concept. Instead, these standards were written to address and engage in an appropriate depth of knowledge, including perspectives into how that knowledge is obtained and where it fits in broader contexts, for students to continue to use and expand their understandings over a lifetime.
Articulation of SEPs, CCCs, and DCIs
| Science and Engineering Practices |
Crosscutting Concepts |
Disciplinary Core Ideas |
Asking questions or defining problems:
Students engage in asking testable questions and defining problems to pursue understandings of phenomena.
Developing and using models:
Students develop physical, conceptual, and other models to represent relationships, explain mechanisms, and predict outcomes.
Planning and carrying out investigations:
Students plan and conduct scientific investigations in order to test, revise, or develop explanations.
Analyzing and interpreting data:
Students analyze various types of data in order to create valid interpretations or to assess claims/conclusions.
Using mathematics and computational thinking:
Students use fundamental tools in science to compute relationships and interpret results.
Constructing explanations and designing solutions:
Students construct explanations about the world and design solutions to problems using observations that are consistent with current evidence and scientific principles.
Engaging in argument from evidence:
Students support their best explanations with lines of reasoning using evidence to defend their claims.
Obtaining, evaluating, and communicating information:
Students obtain, evaluate, and derive meaning from scientific information or presented evidence using appropriate scientific language. They communicate their findings clearly and persuasively in a variety of ways including written text, graphs, diagrams, charts, tables, or orally. |
Patterns:
Students observe patterns to organize and classify factors that influence relationships
Cause and effect:
Students investigate and explain causal relationships in order to make tests and predictions.
Scale, proportion, and quantity:
Students compare the scale, proportions, and quantities of measurements within and between various systems.
Systems and system models:
Students use models to explain the parameters and relationships that describe complex systems.
Energy and matter:
Students describe cycling of matter and flow of energy through systems, including transfer, transformation, and conservation of energy and matter.
Structure and function:
Students relate the shape and structure of an object or living thing to its properties and functions.
Stability and change:
Students evaluate how and why a natural or constructed system can change or remain stable over time. |
Physical Sciences:
(PS1) Matter and Its Interactions
(PS2) Motion and Stability: Forces and Interactions
(PS3) Energy
(PS4) Waves
Life Sciences:
(LS1) Molecules to Organisms
(LS2) Ecosystems
(LS3) Heredity
(LS4) Biological Evolution
Earth and Space Sciences:
(ESS1) Earth’s Place in the Universe
(ESS2) Earth’s Systems
(ESS3) Earth and Human Activity
Engineering Design:
(ETS1.A) Defining and Delimiting an Engineering Problem
(ETS1.B) Developing Possible Solutions
(ETS1.C) Optimizing the Design Solution |
Organization of Standards
The Utah SEEd standards are organized into strands which represent significant areas of learning within grade level progressions and content areas. Each strand introduction is an orientation for the teacher in order to provide an overall view of the concepts needed for foundational understanding. These include descriptions of how the standards tie together thematically and which DCIs are used to unite that theme. Within each strand are standards. A standard is an articulation of how a learner may demonstrate their proficiency, incorporating not only the disciplinary core idea but also a crosscutting concept and a science and engineering practice. While a standard represents an essential element of what is expected, it does not dictate curriculum—it only represents a proficiency level for that grade. While some standards within a strand may be more comprehensive than others, all standards are essential for a comprehensive understanding of a strand’s purpose.
The standards of any given grade or course are not independent. SEEd standards are written with developmental levels and learning progressions in mind so that many topics are built upon from one grade to another. In addition, SEPs and CCCs are especially well paralleled with other disciplines, including English language arts, fine arts, mathematics, and social sciences. Therefore, SEEd standards should be considered to exist not as an island unto themselves, but as a part of an integrated, comprehensive, and holistic educational experience.
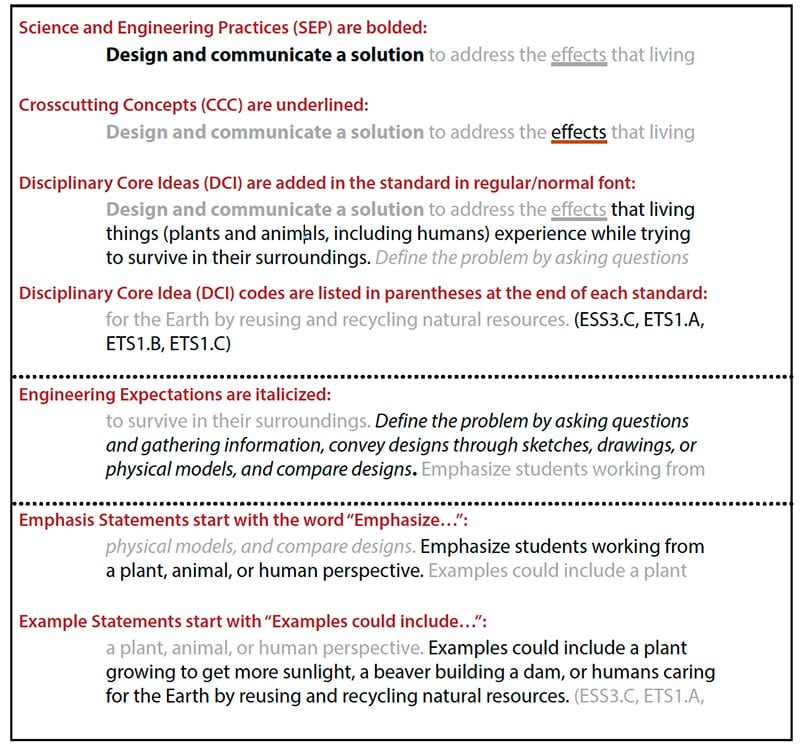
Each standard is framed upon the three dimensions of science to represent a cohesive, multi-faceted science learning outcome.
- Within each SEEd Standard Science and Engineering Practices are bolded.
- Crosscutting Concepts are underlined.
- Disciplinary Core Ideas are added to the standard in normal font with the relevant DCIs codes from the K–12 Framework (indicated in parentheses after each standard) to provide further clarity.
- Standards with specific engineering expectations are italicized.
- Many standards contain additional emphasis and example statements that clarify the learning goals for students.
- Emphasis statements highlight a required and necessary part of the student learning to satisfy that standard.
- Example statements help to clarify the meaning of the standard and are not required for instruction.
An example of a SEEd standard:
- Standard K.2.4 Design and communicate a solution to address the effects that living things (plants and animals, including humans) experience while trying to survive in their surroundings. Define the problem by asking questions and gathering information, convey designs through sketches, drawings, or physical models, and compare designs. Emphasize students working from a plant, animal, or human perspective. Examples could include a plant growing to get more sunlight, a beaver building a dam, or humans caring for the Earth by reusing and recycling natural resources. (ESS3.C, ETS1.A, ETS1.B, ETS1.C)
Each part of the above SEEd standard is identified in the following diagram:
Goal of the SEEd Standards
The Utah SEEd Standards is a research-grounded document aimed at providing accurate and appropriate guidance for educators and stakeholders. But above all else, the goal of this document is to provide students with the education they deserve, honoring their abilities, their potential, and their right to utilize scientific thought and skills for themselves and the world that they will build.
1 National Research Council. 2012. A Framework for K–12 Science Education: Practices, Crosscutting Concepts, and Core Ideas. Washington, DC: The National Academies Press. https://doi.org/10.17226/13165. This consensus research document and its chapters are referred to throughout this document as a research basis for much of Utah’s SEEd standards.
2 Most Utah SEEd Standards are based on the Next Generation Science Standards (NGSS Lead States. 2013. Next Generation Science Standards: For States, By States. Washington, DC: The National Academies Press) http://www. nextgenscience.org
Introduction
The chemistry SEEd standards explore the foundational principles of chemistry that allow students to investigate the ways in which chemistry impacts everyday life. Students inves- tigate the properties and structure of matter at atomic and subatomic scales to explain how they influence a system’s larger scale, structures, properties, and functions. Students explain how macroscopic observations are translated into molecular-level representations and then develop and use these models to describe molecules with chemical equations
or mathematical expressions. Students analyze data on the relationships between atomic and molecular structures and the properties of materials that are observed macroscopi- cally using the human senses and scientific instruments. Students explain that matter is conserved in chemical reactions and nutrient cycles, the ability of humans to design and control chemical systems for the benefit of society, and the ways that energy interacts with matter. Additionally, students design and evaluate solutions to problems that exist in these areas.
Core Standards of the Course
Strand CHEM.1: THE STRUCTURE AND PROPERTIES OF ATOMS
Atoms have substructures of their own including a small central nucleus containing protons and neutrons surrounded by a larger region containing electrons. The strong nuclear interaction provides the primary force that holds nuclei together. Without it, the electromagnetic forces between protons would make all nuclei other than hydrogen unstable. Processes of fusion, fission, and radioactive decay of unstable nuclei involve changes in nuclear binding energies. Elements are placed in columns and rows on the periodic table to reflect their common and repeating properties.
Standard CHEM.1.1
Obtain, evaluate, and communicate information regarding the structure of the atom on the basis of experimental evidence. Emphasize the relationship between proton number and element identity, isotopes, and electrons in atoms. Examples of experimental evidence could include the gold foil experiment, cathode ray tube, or atomic spectrum data. (PS1.A)
Standard CHEM.1.2
Analyze and interpret data to identify patterns in the stability of isotopes and predict likely modes of radioactive decay. Emphasize that different isotopes of the same element decay by different modes and at different rates depending on their nuclear stability. Examples of data could include band of stability charts, mass or nuclear binding energy per nucleon, or the inverse relationship between half-life and nuclear stability. (PS1.C)
Standard CHEM.1.3
Use mathematics and computational thinking to relate the rates of change in quantities of radioactive isotopes through radioactive decay (alpha, beta, and positron) to ages of materials or persistence in the environment. Emphasize a conceptual understanding of half-life. Examples could include radiocarbon dating, nuclear waste management, or nuclear medicine. (PS1.C)
Standard CHEM.1.4
Construct an explanation about how fusion can form new elements with greater or lesser nuclear stability. Emphasize the nuclear binding energy, with the conceptual understanding that when fusion of elements results in a more stable nucleus, large quantities of energy are released, and when fusion results in a less stable nucleus, large quantities of energy are required. Examples could include the building up of elements in the universe starting with hydrogen to form heavier elements, the composition of stars, or supernovae producing heavy elements. (PS1.C, ESS1.A)
Standard CHEM.1.5
Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms. Emphasize conceptual understanding of trends and patterns. Examples could include trends in ionization energy, atomic radius, or electronegativity. Examples of properties for main group elements could include general reactivity, bonding type, or ion formation. (PS1.A)
Strand CHEM.2: THE STRUCTURE AND PROPERTIES OF MOLECULES
Electrical attractions and repulsions between charged particles (atomic nuclei and electrons) in matter explain the structure of atoms and the forces between atoms that cause them to form molecules via chemical bonds. Molecules can range in size from two atoms to thousands of atoms. The same forces cause atoms to combine to form extended structures, such as crystals or metals. The varied properties of the materials, both natural and manufactured, can be understood in terms of the atomic and molecular particles present and the forces within and between them. Materials are engineered to fulfill a desired function or role with desired properties.
Standard CHEM.2.1
Analyze data to predict the type of bonding most likely to occur between two elements using the patterns of reactivity on the periodic table. Emphasize the types and strengths of attractions between charged particles in ionic, covalent, and metallic bonds. Examples could include the attraction between electrons on one atom and the nucleus of another atom in a covalent bond or between ions in an ionic compound. (PS1.A, PS2.B)

Standard CHEM.2.2
Plan and carry out an investigation to compare the properties of substances at the bulk scale and relate them to molecular structures. Emphasize using models to explain or describe the strength of electrical forces between particles. Examples of models could include Lewis dot structures or ball and stick models. Examples of particles could include ions, atoms, molecules, or networked materials (such as graphite). Examples of properties could include melting point and boiling point, vapor pressure, solubility, or surface tension. (PS1.A)
Standard CHEM.2.3
Engage in argument supported by evidence that the functions of natural and designed macromolecules are related to their chemical structures. Emphasize the roles of attractive forces between and within molecules. Examples could include non-covalent interactions between base pairs in DNA allowing it to be unzipped for replication, the network of atoms in a diamond conferring hardness, or the nonpolar nature of polyester (PET) making it quick-drying. (PS1.A)
Standard CHEM.2.4
Evaluate design solutions where synthetic chemistry was used to solve a problem (cause and effect). Define the problem, identify criteria and constraints, analyze available data on proposed solutions, and determine an optimal solution. Emphasize the design of materials to control their properties through chemistry. Examples could include pharmaceuticals that target active sites, teflon to reduce friction on surfaces, or nanoparticles of zinc oxide to create transparent sunscreen. (PS1.A, ETS1.A, ETS1.B, ETS1.C)
Strand CHEM.3: STABILITY AND CHANGE IN CHEMICAL SYSTEMS
Conservation of matter describes the cycling of matter and the use of resources. In both chemical and physical changes, the total number of each type of atom is conserved. When substances are combined, they may interact with each other to form a solution. The proportion of substances in a solution can be represented with concentration. In a chemical change, the atoms are rearranged by breaking and forming bonds to create different molecules, which may have different properties. Chemical processes can be understood in terms of the collisions of molecules and the rearrangements of atoms. The rate at which chemical processes occur can be modified. In many situations, a dynamic and condition-dependent balance between a reaction and the reverse reaction determines the numbers of all types of molecules present. Chemists can control and design chemical systems to create desirable results, although sometimes there are also unintended consequences.
Standard CHEM.3.1
Use mathematics and computational thinking to analyze the distribution and proportion of particles in solution. Emphasize proportional reasoning and the impact of concentration on solution properties, rather than algorithmic calculations. Examples of concentrations affecting solutions could include the Beer-Lambert Law, colligative properties, or pH. (PS1.A)
Standard CHEM.3.2
Analyze data to identify patterns that assist in making predictions of the outcomes of simple chemical reactions. Emphasize patterns based on the outermost electrons of atoms, trends in the periodic table, and knowledge of chemical properties. Examples could include reactions between main group elements, combustion reactions, or reactions between Arrhenius acids and bases. (PS1.B)
Standard CHEM.3.3
Plan and carry out an investigation to observe the change in properties of substances in a chemical reaction to relate the macroscopically observed properties to the molecular level changes in bonds and the symbolic notation used in chemistry. Emphasize that the visible macroscopic changes in chemical reactions are a result of changes on the molecular level. Examples of observable properties could include changes in color or the production of a solid or gaseous product. (PS1.B)
Standard CHEM.3.4
Use mathematics and computational thinking to support the observation that matter is conserved during chemical reactions and matter cycles. Emphasize that chemical reactions occur on both small and global scales, and that matter is always conserved. Examples of small scale reactions could include ratios of reactants and products in a single chemical reaction or simple stoichiometric calculation. Examples of global scale matter cycles could include tracing carbon through the chemical reactions of photosynthesis, combustion, or respiration. (PS1.B)
Standard CHEM.3.5
Develop solutions related to the management, conservation, and utilization of mineral resources (matter). Define the problem, identify criteria and constraints, develop possible solutions using models, analyze data to make improvements from iteratively testing solutions, and optimize a solution. Emphasize the conservation of matter and minerals as a limited resource. Examples of Utah mineral resources could include copper, uranium, potash, coal, oil, or natural gas. Examples of constraints could include cost, safety, reliability, or possible social, cultural, and environmental impacts. (PS1.B, ESS3.A, ETS1.A, ETS1.B, ETS1.C)

Standard CHEM.3.6
Construct an explanation using experimental evidence for how reaction conditions affect the rate of change of a reaction. Emphasize collision theory as an explanatory principle. Examples of reaction conditions could include temperature, concentration, particle size, or presence of a catalyst. (PS1.B)
Standard CHEM.3.7
Design a solution that would refine a chemical system by specifying a change in conditions that would produce increased or decreased amounts of a product at equilibrium. Define the problem, identify criteria and constraints, develop possible solutions using models, analyze data to make improvements from iteratively testing solutions, and optimize a solution. Emphasize a qualitative understanding of Le Châtelier's Principle and connections between macroscopic and molecular level changes. (PS1.B, ETS1.A, ETS1.B, ETS1.C)
Standard CHEM.3.8
Obtain, evaluate, and communicate information regarding the effects of designed chemicals in a complex real-world system. Emphasize the role of chemistry in solving problems, while acknowledging unintended consequences. Examples could include ozone depletion and restoration, DDT, development of medicines, the preservation of historical artifacts, or use of bisphenol-A in plastic manufacturing. (PS1.A)
Strand CHEM.4: ENERGY IN CHEMICAL SYSTEMS
A system's total energy is conserved as energy is continually transferred from one particle to another and between its various possible forms. The energy of a system depends on the motion and interactions of matter and radiation within that system. When bonds are formed between atoms, energy is released. Energy must be provided when bonds are broken. When electromagnetic radiation with longer wavelengths is absorbed by matter, it is generally converted into thermal energy or heat. When visible light is absorbed by matter, it results in phenomena related to color. When shorter wavelength electromagnetic radiation is absorbed by matter, it can ionize atoms and cause damage to living cells. Nuclear processes, including fusion, fission, and radioactive decays of unstable nuclei, involve the release or absorption of large amounts of energy. Society's demand for energy requires thinking creatively about ways to provide energy that don't deplete limited resources or produce harmful emissions.
Standard CHEM.4.1
Construct an argument from evidence about whether a simple chemical reaction absorbs or releases energy. Emphasize that the overall change in energy is related to the energy absorbed when bonds are broken and the energy released when bonds are formed. Examples could include chemical reactions releasing or absorbing energy to or from the surrounding solution or the metabolism of glucose. (PS1.B, PS3.B)
Standard CHEM.4.2
Construct an explanation of the effects that different frequencies of electromagnetic radiation have when absorbed by matter. Emphasize a qualitative understanding. Examples could include that low energy electromagnetic radiation can increase molecular rotation and bond vibration, visible light can cause electronic transitions, and high energy electromagnetic radiation can result in ionization and bond breaking. (PS4.B)
Standard CHEM.4.3
Design a device that converts energy from one form into another to solve a problem. Define the problem, identify criteria and constraints, develop possible solutions using models, analyze data to make improvements from iteratively testing solutions, and optimize a solution. Emphasize chemical potential energy as a type of stored energy. Examples of sources of chemical potential energy could include oxidation-reduction or combustion reactions. (PS3.B, ETS1.A, ETS1.B, ETS1.C)
Standard CHEM.4.4
Use models to describe the changes in the composition of the nucleus of the atom during nuclear processes, and compare the energy released during nuclear processes to the energy released during chemical processes. Emphasize a qualitative understanding of nuclear changes. Examples of nuclear processes could include the formation of elements through fusion in stars, generation of electricity in a nuclear power plant, radioactive decay, or the use of radioisotopes in nuclear medicine. (PS1.C, PS3.D)
Standard CHEM.4.5
Develop an argument from evidence to evaluate a proposed solution to societal energy demands based on prioritized criteria and trade-offs that account for a range of constraints that could include cost, safety, reliability, as well as possible social, cultural, and environmental impacts. (PS3.D, ETS1.A, ETS1.B, ETS1.C)
 http://www.uen.org - in partnership with Utah State Board of Education
(USBE) and Utah System of Higher Education
(USHE). Send questions or comments to USBE
Specialist -
Milo
Maughan
and see the Science - Secondary website. For
general questions about Utah's Core Standards contact the Director
-
Jennifer
Throndsen.
These materials have been produced by and for the teachers of the
State of Utah. Copies of these materials may be freely reproduced
for teacher and classroom use. When distributing these materials,
credit should be given to Utah State Board of Education. These
materials may not be published, in whole or part, or in any other
format, without the written permission of the Utah State Board of
Education, 250 East 500 South, PO Box 144200, Salt Lake City, Utah
84114-4200.
http://www.uen.org - in partnership with Utah State Board of Education
(USBE) and Utah System of Higher Education
(USHE). Send questions or comments to USBE
Specialist -
Milo
Maughan
and see the Science - Secondary website. For
general questions about Utah's Core Standards contact the Director
-
Jennifer
Throndsen.
These materials have been produced by and for the teachers of the
State of Utah. Copies of these materials may be freely reproduced
for teacher and classroom use. When distributing these materials,
credit should be given to Utah State Board of Education. These
materials may not be published, in whole or part, or in any other
format, without the written permission of the Utah State Board of
Education, 250 East 500 South, PO Box 144200, Salt Lake City, Utah
84114-4200.
 Course Introduction
Course Introduction




 UTAH EDUCATION NETWORK
UTAH EDUCATION NETWORK

 Justin
Justin Braxton
Braxton Dani
Dani Kayla
Kayla Katie
Katie Lora
Lora Rob
Rob Val
Val

